GLOBAL CANCER OBSERVATORY
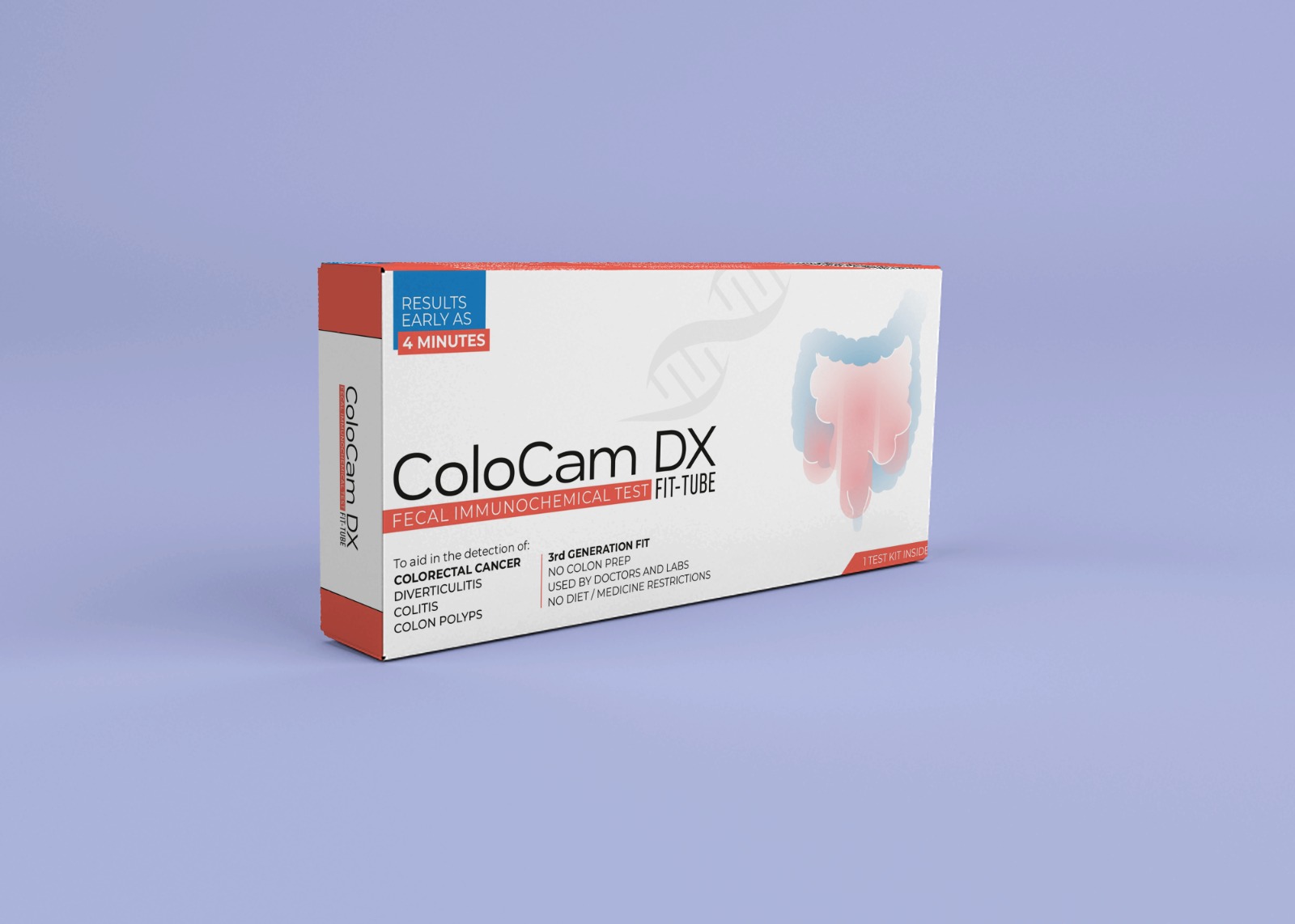
Colocam DX-FIT
Tube
The over-the-counter self-test kit offers unparalleled convenience:

Colocam DX-FIT Tube
ColoCam Tube® is a CDSCO approved advanced, non-invasive Fecal Immunochemical Test (FIT) designed for reliable, accurate, and affordable all colon disease screening including colon cancer. Recommended and trusted by doctors and hospitals worldwide, this test kit makes early detection simple and accessible.
Take control of your colon health with ColoCam Tube®—a convenient and effective solution for early detection.
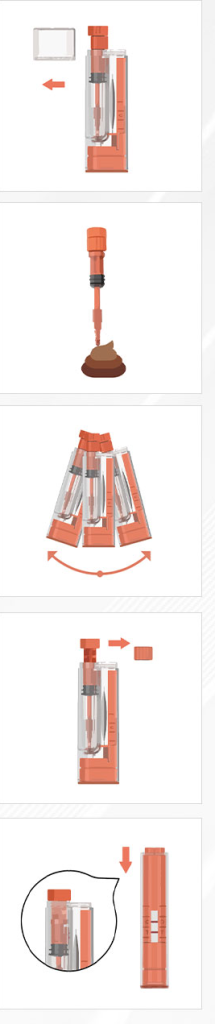
With ColoCam Tube®, you can easily perform the test in the comfort. The process is quick and straightforward, delivering accurate results in just five minutes. No diet control, no prescription, doctor’s visit, or sending of samples to a laboratory is required, making it a hassle-free option for proactive health care.
Redefining Colon Cancer Screening
FAQ
ask us
anything
- FIT (fecal immunochemical test) detects human hemoglobin in stool specifically from the lower gastrointestinal tract, making it more accurate for CRC screening without interference from diet or medications.
- Unlike guaiac-based FOBT (gFOBT), which requires dietary restrictions and has lower sensitivity (50–70%), FIT is preferred for annual screening.
- Compared to multitarget stool DNA tests (e.g., Cologuard), FIT is cheaper, requires no DNA analysis, and has higher specificity (reducing false positives), but lower sensitivity for advanced adenomas (23–40% vs. 42% for DNA tests).
- FIT is ideal for average-risk adults aged 40–75, as recommended by the USPSTF and ACS, especially those hesitant about colonoscopy due to sedation or prep concerns.
- In promotions, gastroenterologists target underserved or low-adherence populations to boost screening rates up to 34% higher than standard care. Avoid FIT for high-risk patients (e.g., family history of CRC, IBD), those with recent colonoscopy (within 10 years) or symptoms like rectal bleeding, or post-inadequate bowel prep colonoscopy (wait 5 years).
- Quantitative FITs are suggested over qualitative for adjustable thresholds.
- No prep is needed—collect a small stool sample (pea-sized) using the probe during a normal bowel movement, avoiding urine contamination (use provided paper)
- Is it safe and easy to use?
- Yes, the kit is designed for at-home testing with clear instructions. There is no need for a doctor’s visit, prescription, or mailing samples to a lab. Results are typically available within five minutes.
- OTC kits (e.g., Second Generation FIT) give results in 5 minutes at home.
- In promotions, emphasize rapid return to minimize degradation; remind patients via calls to achieve 80–90% completion rates. One sample is often sufficient, but guidelines support 1–2 for higher sensitivity.
- A positive result (blood detected) indicates possible CRC, polyps, or other issues (e.g., hemorrhoids, diverticulitis)—not a diagnosis.
- Recommend colonoscopy within 8 weeks, as per ColonCancerCheck guidelines, to identify the source.
- In promotional contexts, gastroenterologists stress free nurse support or referrals for follow-up to ensure 70–80% completion rates.
- Do not repeat FIT; false positives occur in 5–10% of cases from non-cancer sources.
- Annual testing is recommended for average-risk patients to account for intermittent bleeding (10–20% false negatives).
- A negative result means low CRC risk that year but doesn’t rule out polyps; continue annually.
- In VA or safety-net promotions, programmatic mailed FITs have increased adherence by reminding overdue patients, reducing colonoscopy backlog by prioritizing high-risk cases.
- FIT has 92–98% sensitivity for CRC and 23–40% for advanced adenomas, with fewer false positives than gFOBT.
- Benefits include no sedation, low cost, and high patient acceptance (e.g., OTC kits like Pinnacle BioLabs promoted as “easy alternative” by gastroenterologists).
- Limitations: Misses upper GI bleeds, requires annual use, and positive results need colonoscopy (increasing demand). In promotions, highlight 90% CRC preventability via early detection.
